Why FDA Compliance Is Non-Negotiable for U.S. Food Imports
Exporting instant noodles to the United States requires full compliance with the U.S. Food and Drug Administration (FDA). Missing or incorrect steps can lead to customs rejections, fines, or destroyed shipments.
At Kimdee OEM Services, we support your private label brand through FDA registration, compliant packaging, and seamless exports.
1. Register Your Manufacturing Facility with the FDA
All foreign food facilities must complete FDA registration:
Submit your FDA Food Facility Registration online
Designate a U.S. Agent for correspondence
Renew registration every two years
🔗 FDA Facility Registration Portal
💡 Kimdee ensures your OEM facility is FDA-registered and export-ready.
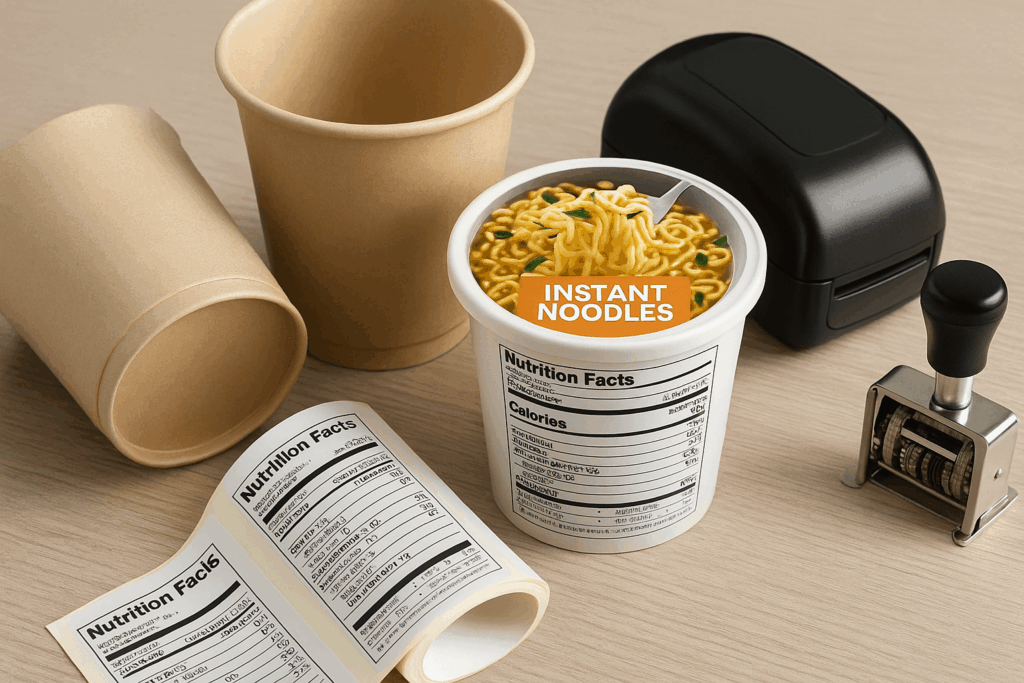
2. Create FDA-Compliant Food Labels
Your packaging must meet U.S. labeling standards:
FDA-format Nutrition Facts Panel
English-only ingredient lists (in descending order)
Proper allergen disclosure (e.g. Contains: Wheat, Soy)
Net weight in grams and ounces
Manufacturer and country of origin
See our guide: How to Label and Package Food for Export
3. Check for FDA Import Alerts Before You Ship
FDA maintains a list of red-flagged companies and ingredients that delay or block imports.
Check here before shipping:
🔗 FDA Import Alerts List
Common triggers:
Missing facility registration
Incorrect nutrition facts format
Undeclared allergens
Unsanitary manufacturing reports
4. File Prior Notice and Work with a Licensed U.S. Customs Broker
Before entering U.S. ports:
Submit a Prior Notice to FDA
Partner with a customs broker who understands FDA entries
Keep documents ready: invoice, packing list, labels, registration ID
5. Partner with FDA-Compliant OEM Manufacturers
Working with the right OEM partner reduces your risk.
✅ Kimdee’s production facilities are:
FDA registered
Operated under GMP & HACCP protocols
Export-ready with fast compliance documentation
Capable of producing low-MOQ test runs
📩 Need help launching an FDA-compliant product?
Email carlng@kimdeefoods.com or visit our OEM Service Page to get started.
Frequently Asked Questions
Q1: Does my food need to be FDA “approved”?
🅰️ No. Most foods only need to be registered, not “approved.” Approval is required for drugs or supplements.
Q2: How fast can I get my facility registered?
🅰️ If all documents are ready, typically 3–5 business days.
Q3: Can Kimdee help with FDA-compliant label design?
🅰️ Yes. We provide turnkey label templates and handle layout review for compliance.